Belgium’s Tigenix says heart attack stem cell trial successful – Reuters
By JoanneRUSSELL25
BRUSSELS Belgian biotech group Tigenix said on Monday its medical trial with a novel treatment for patients at risk of heart failure after a coronary attack was successful.
The group said patients treated in its PhaseI/II trial of donor-derived expanded cardiac stem cells (AlloCSC) showed no side-effects and all of them continued to live after 30 days, six months and a year.
Tigenix added that in one subgroup of trial patients associated with a poor long-term outlook, there was a larger reduction in the size of infarction, tissue death due to inadequate blood supply.
"This is the first trial in which it has been demonstrated that allogeneic cardiac stem cells can be transplanted safely through the coronary tree," one of the doctors in the trial said.
The group said it would now analyze the data from the trial and decide on how to proceed with its research.
(Reporting by Robert-Jan Bartunek; editing by Philip Blenkinsop)
LONDON A French biotech firm is hoping to gain approval within months for a nuclear medicine targeting the type of cancer that killed Steve Jobs.
NEW YORK Developers of an experimental blood test for autism say it can detect the condition in more than 96 percent of cases and do so across a broad spectrum of patients, potentially allowing for earlier diagnosis, according to a study released on Thursday.
(Reuters Health) - Getting too little sleep in early childhood is linked to cognitive and behavioral problems years later, a U.S. study suggests.
See the article here:
Belgium's Tigenix says heart attack stem cell trial successful - Reuters
Scientists create ‘beating’ human heart muscle for cardiac research – UQ News
By daniellenierenberg
Scientists at The University of Queensland have taken a significant step forward in cardiac disease research by creating a functional beating human heart muscle from stem cells.
Dr James Hudson and Dr Enzo Porrello from the UQ School of Biomedical Sciences collaborated with German researchers to create models of human heart tissue in the laboratory so they can study cardiac biology and diseases in a dish.
The patented technology enables us to now perform experiments on human heart tissue in the lab, Dr Hudson said.
This provides scientists with viable, functioning human heart muscle to work on, to model disease, screen new drugs and investigate heart repair.
The UQCardiac Regeneration Laboratoryco-leaders have also extended this research and shown that the immature tissues have the capacity to regenerate following injury.
In the laboratory we used dry ice to kill part of the tissue while leaving the surrounding muscle healthy and viable, Dr Hudson said.
We found those tissues fully recovered because they were immature and the cells could regenerate in contrast to what happens normally in the adult heart where you get a dead patch.
Our goal is to use this model to potentially find new therapeutic targets to enhance or induce cardiac regeneration in people with heart failure.
Studying regeneration of these damaged, immature cells will enable us to figure out the biochemical events behind this process.
Hopefully we can determine how to replicate this process in adult hearts for cardiovascular patients.
UQ scientists create beating human heart muscle from The University of Queensland on Vimeo.
Each year, about 54,000 Australians suffer a heart attack, with an average of about 23 deaths every day.
The UQ research has been supported by the National Health and Medical Research Council (NHMRC) and the National Heart Foundation.
Heart Foundation Queensland CEO Stephen Vines said the charity was excited to fund such an important research project.
Heart attack survivors who have had permanent damage to their heart tissue are essentially trying to live on half an engine, Mr Vines said.
The research by Dr Hudson and Dr Porello will help unlock the key to regenerating damaged heart tissue, which will have a huge impact on the quality of life for heart attack survivors.
Dr Hudson and Dr Porello are deserved recipients of our highest national research accolade the Future Leader Fellowship Award.
The research is published in Circulation and Development.
Media: Dr James Hudson, j.hudson@uq.edu.au; Kim Lyell, k.lyell@uq.edu.au, 0427 530 647.
Go here to see the original:
Scientists create 'beating' human heart muscle for cardiac research - UQ News
Belgium’s Tigenix says heart attack stem cell trial successful – KFGO
By daniellenierenberg
Monday, March 13, 2017 3 a.m. CDT
BRUSSELS (Reuters) - Belgian biotech group Tigenix said on Monday its medical trial with a novel treatment for patients at risk of heart failure after a coronary attack was successful.
The group said patients treated in its PhaseI/II trial of donor-derived expanded cardiac stem cells (AlloCSC) showed no side-effects and all of them continued to live after 30 days, six months and a year.
Tigenix added that in one subgroup of trial patients associated with a poor long-term outlook, there was a larger reduction in the size of infarction, tissue death due to inadequate blood supply.
"This is the first trial in which it has been demonstrated that allogeneic cardiac stem cells can be transplanted safely through the coronary tree," one of the doctors in the trial said.
The group said it would now analyze the data from the trial and decide on how to proceed with its research.
(Reporting by Robert-Jan Bartunek; editing by Philip Blenkinsop)
Read this article:
Belgium's Tigenix says heart attack stem cell trial successful - KFGO
Daiichi Sankyo forges $12M pact for GPCR pain program with Heptares; Incyte shares jump on latest takeover chatter – Endpoints News
By JoanneRUSSELL25
Heptares struck a $12 million deal to partner with Daiichi Sankyo on a new GPCR pain drug. The UK biotech gets $4 million upfront and $8 million in research support along with an unspecified set of milestones for the deal, in which the Sosei subsidiary will search for new drugs that can be developed for pain. Said CEO Malcolm Weir: We are confident that the unique structural insights of the receptor that our technologies can deliver combined with expertise on its role in pain from the Neurosciences team at Daiichi Sankyo will yield new, differentiated molecules that can be advanced into development.
Everybody loves a good takeover rumor. On Friday, it was Incytes turn again. The biotechs shares jumped Friday on buzz that Gilead was interested in acquiring the company, fast on the heels of an analysts report insisting that Gilead needed to do a deal, fast.
Belgiums TiGenix says that it gained some positive data in a Phase I/II cardiac stem cell study. Investigators say that a pre-specified subset of patients demonstrated a larger reduction in infarct size. This is the first trial in which it has been demonstrated that allogeneic cardiac stem cells can be transplanted safely through the coronary tree, and in the worst possible setting represented by patients with an acute heart attack with left ventricular dysfunction, commented Professor Fernndez-Avils, head of the Department of Cardiology at the Hospital General Universitario Gregorio Maran.
News reports for those who discover, develop, and market drugs. Join 13,500+ biopharma pros who read Endpoints News articles by email every day. Free subscription.
See original here:
Daiichi Sankyo forges $12M pact for GPCR pain program with Heptares; Incyte shares jump on latest takeover chatter - Endpoints News
Woodrose Ventures Corporation Announces Proposed Acquisition … – Marketwired (press release)
By raymumme
VANCOUVER, BRITISH COLUMBIA--(Marketwired - March 13, 2017) -
NOT FOR DISSEMINATION IN THE UNITED STATES
Editors Note: There is a photo associated with this press release.
Woodrose Ventures Corporation (TSX VENTURE:WRS.H) ("Woodrose" or the "Company") is pleased to announce that it has entered into an agreement (the "Agreement") dated March 10, 2017 to acquire all of the shares of Novoheart Holdings Ltd. ("Novoheart"), a global stem cell biotechnology company dedicated to human heart engineering (the "Transaction"). Novoheart develops products and provides services focused on engineering prototypes of bio-artificial human heart tissues and chambers for drug discovery, cardiotoxicity screening, disease modelling and therapeutic applications.
The Transaction will constitute a "reverse-takeover" of Woodrose in accordance with the policies of the TSX Venture Exchange (the "TSXV") and the reactivation of Woodrose, which is currently a NEX-listed issuer.
About Novoheart
Novoheart is a global stem cell biotechnology company headquartered in Hong Kong with R&D Innovation Centres being set up in the United States. Novoheart's mission is to revolutionize drug discovery and the development of heart therapeutics with its range of proprietary bioengineered human heart constructs, collectively known as the MyHeart platform, and to further develop them into transplantable heart grafts for cell-based regenerative therapies with superior safety and efficacy. Its scientific team has pioneered a range of best-in-class bioengineering technologies and constructed the world's first human mini-heart "novoHeart" with which the Novoheart team intends to revolutionize:
1) Pre-clinical drug discovery, cardiotoxicity screening and heart disease modelling;
2) Post-discovery, clinical development of novel therapeutics; and
3) Pre-clinical and clinical development of cell-based cardiac regenerative therapies.
Novoheart's immediate focus is to innovate and accelerate the lengthy, expensive and inefficient drug development process. The development of a new drug candidate typically costs US$2-4bn and takes 10+ years (Tufts Centre for the Study of Drug Development, Tufts CSDD R&D Cost Study 2014) with extremely poor success rates of <1% of initial drug candidates making it to market (Willmann et al. 2008, Nature Reviews Drug Discovery 7, 591-607). The primary cause for drug withdrawal and attrition is heart toxicity. Despite substantial pre-human R&D costs (~30% of the entire process), conventional non-human and non-cardiac cell and animal models are poorly predictive of human responses, leading to false negative and false positive pre-clinical results that compromise the overall successes (Chen et al. 2016, Nature Reviews Cardiology 13, 333-349).
Novoheart's intellectual property portfolio, including the human "heart-in-a-jar" (novoHeart) and other related next-generation technologies of the MyHeart platform (see figure below) are unique solutions that help bridge the gap between pre-clinical and clinical drug trials. The MyHeart platform provides advanced human heart surrogates for pre-screening of drug formulas and the elimination of toxic compounds early on in the drug development process, minimizing the risk towards patients. Significantly, the MyHeart Platform provides real time data on the effects of drug formulations enabling drug development companies to undertake "on-the-fly" reformulation of drug candidates to optimize efficacy and toxicological profiles. With Novoheart's technologies, we aim to significantly reduce pre-clinical R&D time and costs, and importantly, improve trial successes. It is anticipated that drug screening results using Novoheart's human engineered tissues would be accepted as reliable indicators for toxicity and efficacy, thereby qualifying the test compounds for accelerated drug development.
Novoheart adopts a hybrid business model by:
These products and services are designed to significantly reduce the time, cost, and use of animal models, as well as improve patient safety, and facilitate pharmaceutical discovery and development. Novoheart is currently working with leading academic and pharmaceutical partners to innovate drug discovery and toxicity screening protocols. Our targeted clients are pharmaceutical companies, government units, and research institutions.
Novoheart was incorporated in 2014 pursuant to the laws of British Virgin Islands (BVI) and its controlling shareholder is Medera Group Limited, a BVI entity. Novoheart has one wholly owned Hong Kong subsidiary "Novoheart Limited" ("Novoheart Hong Kong") which is the group operating entity.
Novoheart Hong Kong was incorporated in January 2014 by founder and CEO Prof. Ronald Li, with scientific co-founders Prof. Kevin Costa and Prof. Michelle Khine.
Novoheart's foundational technologies are the direct outcome of over 15 years of research effort supported by R&D investments amounting to approximately USD30MM. These research efforts, performed at Johns Hopkins University, Icahn School of Medicine at Mount Sinai, University of California Irvine, University of California Davis, and the University of Hong Kong by our scientific founders, have received major recognitions such as American Heart Association's Best Study of 2005, Ground-breaking Study of 2006, and Late-breaking Studies of 2002, 2003, 2005 and 2007, and the Spirit of Hong Kong Innovating for Good Award in 2015. The "human-heart-in-a-jar" technology was selected by Google's Solve For X as a Moonshot Project in 2015.
Novoheart's scientific founders and advisors are renowned pioneering leaders in the stem cell and cardiac space, with a successful track record in developing and commercializing ground-breaking technologies. In September 2014, Novoheart established its R&D base and office in the Hong Kong Science Park, where it continues to innovate solutions for drug discovery and human heart tissue engineering.
In December 2014, Novoheart signed a strategic partnership with a major global pharmaceutical company (the "Global Pharma Partner") headquartered in New York City to validate the MyHeart platform. The success of this validation process has resulted in follow on income-generating projects.
In January 2015, Novoheart's R&D proposal to develop bio-artificial heart tissues for drug screening received 50/50 matched funding from the Innovation & Technology Commission (ITC) of the Government of Hong Kong, with a total project cost of over HK$21MM over 2 years. It was also the largest biotech project granted by ITC for that year. Novoheart owns all of the intellectual property generated from this project, and as a result of the R&D, Novoheart has applied or is in the application process for 3 new patents covering newly developed technology, including the human ventricular cardiac anisotropic sheet (hvCAS) as a powerful tool for detecting drug-induced arrhythmias with the results published in the prestigious international peer-reviewed bioengineering journal Advanced Materials (Shum et al. 2017, Advanced Materials, 29). Additionally, Novoheart holds exclusive worldwide licenses or options to acquire the same for technologies that constitute its MyHeart platform and future developments.
In December 2015, Novoheart signed a second contract with the Global Pharma Partner to build disease-specific engineered human heart tissues and chambers for drug discovery. The total project cost is US$726,000 over 1.5 years.
In February 2017, the Corporate Venture Fund (CVF) of the Hong Kong Science and Technology Parks Corporation (HKSTPC) completed an equity investment of approximately US$250,000 into Novoheart and an additional investment would be made at the Transaction.
Novoheart Financial Information
The following table includes a summary of certain financial information of Novoheart and is derived from its financial statements for the years ended June 30, 2016 and June 30, 2015.
Summary of the Transaction
Under the terms of the Agreement, the shareholders of Novoheart will receive an aggregate of 66,086,600 common shares of Woodrose on a post-Consolidation basis (see below) ("Woodrose Post-Consolidation Shares"). In addition, a finder's fee of 2,313,038 Woodrose Post-Consolidation Shares will be paid to Cynosure Private Equity Limited in connection with the Transaction.
In connection with the Transaction, Woodrose intends to complete a consolidation of all its outstanding common shares on the basis of 3.56878449 old common shares for each one new common share (the "Consolidation"). In addition, Woodrose intends to complete a non-brokered private placement (the "Private Placement") of 11,700,000 subscription receipts ("Subscription Receipts") at a price of CDN$0.50 per Subscription Receipt to raise gross proceeds of CDN$5,850,000, which will be held in escrow in accordance with the terms of a subscription receipt agreement (the "Subscription Receipt Agreement"). It is anticipated that the Subscription Receipt Agreement will provide that, upon completion of the Transaction, each Subscription Receipt will automatically convert into one Woodrose Post-Consolidation Share. The Subscription Receipt Agreement will also provide that, in the event the Transaction is terminated or does not complete within an agreed timeframe, the Subscription Receipts will be cancelled and the funds will be returned to the holders. Woodrose may pay cash fees in an amount not to exceed 7% of the gross proceeds (to a maximum of $364,000) to certain finders involved in the Private Placement and may issue finder's warrants ("Finder's Warrants"), in an amount not to exceed 7% of the number of Subscription Receipts issued (to a maximum of 728,000 Finders Warrants) each of which would entitle the holder to acquire one Woodrose Post-Consolidation Share at a price of CDN$0.50 for a period of two years following closing of the Private Placement. All securities issued pursuant to the Private Placement will be subject to a statutory hold period of four months and one day.
The Company intends to use the net proceeds of the offering to finance investment in drug discovery and screening, establish commercial partnerships, expand the current laboratory, hire additional research and development team members and for working capital and general corporate purposes.
Upon completion of the Transaction, it is anticipated that the Company will be classified as a Tier 2 Technology Issuer on the TSXV and will change its name to "Novoheart Holdings (BC) Limited" or such other name as is acceptable to the Board of Directors. Closing of the Transaction ("Closing") is subject to conditions precedent, that include, but are not limited to, the following:
The Transaction is an "arm's length" transaction (as defined by the policies of the TSXV). Woodrose intends to rely an exemption from the sponsorship requirements of the policies of the TSXV.
Proposed Management Team
Upon closing of the Transaction, the following directors and senior officers are anticipated to be appointed in replacement of Woodrose's current board and management:
Prof. Ronald Li, B.Sc. (Hons), Ph.D. (Proposed President, Chief Executive Officer and Director)
Prof. Ronald Li is a co-founder of Novoheart, and has been serving as the CEO since 2016. He is concurrently Director of Ming-Wai Lau Centre for Reparative Medicine, HK node, Karolinska Institutet (KI), Sweden, with a professorial cross appointment at the Dr. Li Dak-Sum Research Centre, The University of Hong Kong (HKU)-KI Collaboration in Regenerative Medicine of HKU. Prof. Li has been an advocate of stem cell technology for many years, starting from his career as Assistant Professor of Cardiology, and Cellular and Molecular Medicine at the Johns Hopkins University (JHU) School of Medicine. He founded and led the Human Embryonic Stem Cell Consortium when he was recruited in 2005 to become a tenured Associate Professor at the University of California, Davis, in light of state's USD3-billion stem cell initiative Proposition 71. Prof. Li was the Founding Director of the Stem Cell & Regenerative Medicine Consortium (SCRMC) at the University of Hong Kong (HKU) from 2010 to 2015. He also co-directed the Section of Cardiovascular Cell & Tissue Engineering in Icahn School of Medicine at Mount Sinai with Prof. Kevin Costa. Prof. Li has received multiple accolades and recognitions during his career, including the Spirit of Hong Kong Innovating for Good Award by the South China Morning Post (2015), the Top Young Faculty Award (2002, 2004), the Top Prize for the Young Investigator Basic Research (2001) and Top Postdoctoral Fellow Helen Taussig Award (2001) of JHU School of Medicine, Young Investigator Award 1st Prize from the Heart Rhythm Society (2002), and the Career Development Award from the Cardiac Arrhythmias Research & Education Foundation (2001).
Prof. Li graduated with his B.S. with honors in Biotechnology from University of Waterloo, Ontario, on Dean's List and his Ph.D. in Cardiology/Physiology at the University of Toronto.
Dr. Camie Chan, B.Sc. (Hons), M.Sc., Ph.D. (Proposed Chief Operating Officer and Director)
Dr. Camie Chan joined Novoheart Hong Kong as the Chief Operating Officer in 2016, after having served at HKU as the Deputy Director of the Faculty of Medicine Core Facility, a founding member of the Management Committee of the Stem Cell & Regenerative Medicine Consortium (SCRMC), and Assistant Professor in the Department of Anatomy, between 2010 and 2016. She has had extensive experience managing laboratory operations in her capacity at HKU, and her prior career as Assistant Professor at the University of California, Davis, and Assistant Investigator at the Shriners Hospital for Children. Dr. Chan is also a co-inventor of technology allowing mass production of human ventricular heart cells from pluripotent stem cells.
Dr. Chan graduated with her B.Sc. with honors at the University of Waterloo, followed by obtaining her M.Sc. degree in Medical Sciences and Ph.D. degree in Immunology at the University of Toronto, Canada. She then received postdoctoral training at the Sydney Kimmel Cancer Research Center at the Johns Hopkins University. She has garnered numerous awards in her career, including the prestigious National Institute of Allergy and Infectious Diseases (NIAID) Developmental Research Grant Award.
Prof. Kevin Costa, B.S., Ph.D. (Proposed Chief Scientific Officer)
Prof. Costa is Director of the Section of Cardiovascular Cell and Tissue Engineering at the Icahn School of Medicine at Mount Sinai in New York City. Prof. Costa was previously trained at the Johns Hopkins University and on the faculty as Associate Professor of Biomedical Engineering at Columbia University. As a "blue-blood" biomedical engineering (BME) expert (B.S. and M.S. in BME from Boston University, Ph.D. in BME from UC San Diego, and postdoc in BME from JHU and University of Washington) in cell and tissue biomechanics and cardiac tissue engineering, he has developed one of the first engineered cardiac tissue systems. Since 2009, he has been working with Prof. Ronald Li to translate such systems into human cells. Prof. Costa has received research funding from the Whitaker Foundation, the National Science Foundation (NSF) and the National Institutes of Health (NIH; NHLBI, NIBIB, and NIGMS). He was also a recipient of the prestigious Faculty Early Career Development (CAREER) Award from the NSF. Prof. Costa is an inventor of several cardiac tissue engineering technologies and one of the scientific co-founders of Novoheart Hong Kong.
Ms. Iris Lo, B. Comm. (Hons), CPA, CA (Proposed Chief Financial Officer)
Ms. Lo is a seasoned professional with expertise in corporate finance, mergers and acquisitions, accounting, and finance. Prior to joining Novoheart, Ms. Lo was the Director of Corporate Development & Analysis at Cardiome Pharma Corp., a Canadian public company dually listed on the TSX and NASDAQ (TSX: COM, NASDAQ: CRME). At Cardiome, she held responsibilities in equity and debt financing, corporate mergers and acquisitions, product licensing and distributions, financial planning and analysis, as well as regulatory and risk management. During her tenure at Cardiome, Ms. Lo participated in transactions totaling over US$240 million as Cardiome grew from a company with a market capitalization of US$25 million to over US$150 million at its peak. She brings with her valuable experience from the life sciences and pharmaceutical sector, as well as expertise in dealing with the complexities of operating and financing public corporations. Ms. Lo was also previously a Manager in the Transaction Services team at PwC Hong Kong and began her career articling with KPMG Vancouver. She is a Chartered Professional Accountant and holds a Bachelor of Commerce (Honours) from the Sauder School of Business at the University of British Columbia.
Mr. Victor Chang (Proposed Director)
Mr. Chang is a seasoned investor who has lately become focused on start-ups. Mr. Chang started his career with Lippo Securities Limited in 1996 and became a Director of Grand International Holdings Limited in 1999, which was engaged in general investments. During the period from 2007 to 2009, he was a Director and Responsible Officer for Astrum Capital Management Limited carrying out regulated activities under the Securities and Futures Ordinance ("SFO", Cap. 571, Laws of Hong Kong) and with Murtsa Capital Partners Limited as well. During the period from 2007 to 2012, he was also a compliance consultant for Astrum Capital Management Limited. As co-founder and Managing Director of Zebra Strategic Outsource Solution, he has over 16 years of experience in recruitment process outsourcing, executive search as well as and private investment management. In Apil 2013, he successfully brought Zebra Strategic Holdings Limited which offers holistic HR solutions to IPO on the HK GEM board (Stock Code: 8260) and was re-designated as and is currently a Non-Executive Director with the company. He is currently a Director and Responsible Officer of Dakin Financial Group, a corporation licensed to carry out type 1, 2 & 9 regulated activity under the Hong Kong Securities and Futures Ordinance.
Mr. Tong Ricky Chiu (Proposed Director)
As a key founder and visionary for Grand Power Logistics Group Inc., which was listed on the TSX Venture Exchange (GPW.V) before its privatization in 2016, and Baoshinn International Express Ltd., Mr. Chiu adds value with his immense corporate development and growth skills. He received his education in Oxford University, England, and Beijing University, and began his career in Australia. He has a diversified background in a wide range of industries with roles in finance, audit, real estate, merchandise trading and travel, as well as logistics.
Mr. James Topham (Proposed Director)
Mr. Topham is an experienced executive with expertise in finance, accounting, auditing and entrepreneurial technology companies. He was an audit partner leading KPMG's Technology Group in the Vancouver office for 20 years where he worked with many fast growing public companies and was involved in many M&A and IPO transactions in Canada, the US and Europe. Mr. Topham founded Social Venture Partners Vancouver in 2001 with a mission to strengthen the organizational capacity of innovative non-profits serving children in-need and youth at-risk. It has funded several million dollars and provided thousands of hours of executive time mentoring these local non-profits. Since retiring at KPMG 7 years ago, Mr. Topham has worked on several Boards of both public and private technology companies. He received a lifetime achievement award from the BC Technology Industry Association and was awarded the designation of Fellow Chartered Public Accountant (FCPA) from the Chartered Public Accountants of BC for his career achievements in the profession and community. He was a founder and Board member for 9 years of the BC Technology Industry Association that represents the technology industry in BC. Mr. Topham is a CPA and has a Bachelor of Commerce degree with Honours from the University of Saskatchewan graduating as the most distinguished graduate in the College of Commerce.
Mr. Allen Ma (Proposed Director)
As a 30-year technology industry veteran, Mr. Ma was the CEO of Hong Kong Science & Technology Parks before he retired in July 2016. He held senior executive positions within the information and communications technology sector. His past roles include president for Asia-Pacific at British Telecom, vice-president for Asia at the global telecom solutions sector of Motorola, executive director of Hong Kong Telecommunications - subsequently called Cable & Wireless HKT - and managing director of Hong Kong Telecom CSL. Ma holds an MBA from the University of Toronto and is a fellow member of both the Chartered Institute of Management Accountants, UK and the Association of Chartered Certified Accountants, UK. He is also a Certified Management Accountant of Canada.
Proposed Advisory Team
Novoheart is supported by a Scientific Advisory Board whose proposed composition consists of eminent scientists renowned in the fields of stem cells, cardiac biology and physiology, tissue engineering, and clinical cardiology including clinical trials research, from top academic research institutes in the U.S.A. Their technical expertise will guide the development of Novoheart as a forerunner in the application of cutting-edge technologies to develop new and better treatments for heart disease and beyond.
Further Details
Both the Company and Novoheart intend to work diligently to complete the conditions precedent to Closing and anticipate completion of the Transaction in the second quarter of 2017. The Company will update its shareholders with further details as they become available.
ON BEHALF OF WOODROSE VENTURES CORPORATION
Darren Devine, President, CEO and Director
NEITHER THE TSX VENTURE EXCHANGE NOR ITS REGULATION SERVICES PROVIDER (AS THAT TERM IS DEFINED IN THE POLICIES OF THE TSX VENTURE EXCHANGE) ACCEPTS RESPONSIBILITY FOR THE ADEQUACY OR ACCURACY OF THIS RELEASE.
Completion of the Transaction is subject to a number of conditions, including but not limited to, Exchange acceptance and if applicable pursuant to Exchange requirements, majority shareholder approval. Where applicable, the Transaction cannot close until the required shareholder approval is obtained. There can be no assurance that the Transaction will be completed as proposed or at all.
Investors are cautioned that, except as disclosed in the Filing Statement to be prepared in connection with the Transaction, any information with respect to the Transaction may not be accurate or complete and should not be relied on. Trading in securities of the Company should be considered highly speculative.
The TSX Venture Exchange has in no way passed upon the merits of the Transaction and has neither approved nor disproved the contents of this news release.
Cautionary Note Regarding Forward-Looking Statements
Information set forth in this news release may involve forward-looking statements under applicable securities laws. Forward-looking statements are statements that relate to future, not past, events. In this context, forward-looking statements often address expected future business and financial performance, and often contain words such as "anticipate", "believe", "plan", "estimate", "expect", and "intend", statements that an action or event "may", "might", "could", "should", or "will" be taken or occur, or other similar expressions. All statements, other than statements of historical fact, included herein including, without limitation; statements about the terms and completion of the Transaction are forward-looking statements. By their nature, forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements, or other future events, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. Such factors include, among others, the following risks: failure to satisfy all conditions precedent to the Transaction, including shareholder approval, approval of the TSX Venture Exchange and completion of the necessary financings and the additional risks identified in the management discussion and analysis section of Woodrose Corporation's interim and most recent annual financial statement or other reports and filings with the TSX Venture Exchange and applicable Canadian securities regulators. Forward-looking statements are made based on management's beliefs, estimates and opinions on the date that statements are made and the respective companies undertakes no obligation to update forward-looking statements if these beliefs, estimates and opinions or other circumstances should change, except as required by applicable securities laws. Investors are cautioned against attributing undue certainty to forward-looking statements.
To view the photo associated with this press release, please visit the following link: http://www.marketwire.com/library/20170312-1088577_MyHeart_800.jpg
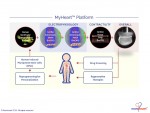
Read the original:
Woodrose Ventures Corporation Announces Proposed Acquisition ... - Marketwired (press release)
VistaGen Therapeutics Inc. (Nasdaq: VTGN) to Ring The Nasdaq Stock Market Closing Bell – GlobeNewswire (press release)
By LizaAVILA
March 10, 2017 15:16 ET | Source: NASDAQ, Inc.
ADVISORY, March 10, 2017 (GLOBE NEWSWIRE) --
What:VistaGen Therapeutics Inc. (Nasdaq:VTGN), a clinical-stage biopharmaceutical company focused on developing new generation medicines for depression and other central nervous system (CNS) disorders, will visit the Nasdaq MarketSite in Times Square.
In honor of the occasion, Shawn K. Singh, CEO & Director, will ring the Closing Bell.
Where:Nasdaq MarketSite 4 Times Square 43rd & Broadway Broadcast Studio
When:Monday, March 13, 2017 3:45 p.m. to 4:00 p.m. ET
VistaGen Contact:Mark A. McPartland (650) 577-3600 IR@vistagen.com
Nasdaq MarketSite:Emily Pan (646) 441-5120 emily.pan@nasdaq.com
Feed Information:Fiber Line (Encompass Waterfront): 4463
Gal 3C/06C 95.05 degrees West 18 mhz Lower DL 3811 Vertical FEC 3/4 SR 13.235 DR 18.295411 MOD 4:2:0 DVBS QPSK
Social Media:For multimedia features such as exclusive content, photo postings, status updates and video of bell ceremonies, please visit our Facebook page: http://www.facebook.com/NASDAQ.
For photos from ceremonies and events, please visit our Instagram page: http://instagram.com/nasdaq
For livestream of ceremonies and events, please visit our YouTube page: http://www.youtube.com/nasdaq/live
For news tweets, please visit our Twitter page: http://twitter.com/nasdaq
For exciting viral content and ceremony photos, please visit our Tumblr page: http://nasdaq.tumblr.com/
Webcast: A live stream of the Nasdaq Closing Bell will be available at: https://new.livestream.com/nasdaq/live or http://www.nasdaq.com/about/marketsitetowervideo.asx
Photos: To obtain a hi-resolution photograph of the Market Close, please go to http://business.nasdaq.com/discover/market-bell-ceremonies and click on the market close of your choice.
About VistaGenVistaGen Therapeutics, Inc.(NASDAQ:VTGN), is a clinical-stage biopharmaceutical company focused on developing new generation medicines for depression and other central nervous system (CNS) disorders. VistaGen's lead CNS product candidate, AV-101, is a new generation oral antidepressant drug candidate in Phase 2 development. AV-101's mechanism of action is fundamentally differentiated from all FDA-approved antidepressants and atypical antipsychotics used adjunctively to treat MDD, with potential to drive a paradigm shift towards a new generation of safer and faster-acting antidepressants. AV-101 is currently being evaluated by the U.S. National Institute of Mental Health (NIMH)in a Phase 2a monotherapy study in MDD being fully funded by the NIMH and conducted by Dr. Carlos Zarate Jr., Chief, Section on the Neurobiology and Treatment of Mood Disorders and Chief of Experimental Therapeutics and Pathophysiology Branch at the NIMH. VistaGen is preparing to launch a 280-patient Phase 2b study of AV-101 as an adjunctive treatment for MDD patients with inadequate response to standard, FDA-approved antidepressant therapies. Dr. Maurizio Fava of Harvard University will be the Principal Investigator of the Phase 2b study. AV-101 may also have the potential to treat multiple CNS disorders and neurodegenerative diseases in addition to MDD, including chronic neuropathic pain, epilepsy, Parkinson's disease and Huntington's disease, where modulation of the NMDAR, AMPA pathway and/or key active metabolites of AV-101 may achieve therapeutic benefit.
VistaStem Therapeutics is VistaGen's wholly owned subsidiary focused on applying human pluripotent stem cell(hPSC)technology, internally and with third-party collaborators, to discover, rescue, develop and commercialize proprietary new chemical entities(NCEs),including small molecule NCEs with regenerative potential, for CNS and other diseases, and cellular therapies involving stem cell-derived blood, cartilage, heart and liver cells. In December 2016, VistaGen exclusively sublicensed to BlueRock Therapeutics LP, a next generation regenerative medicine company established by Bayer AG and Versant Ventures, rights to certain proprietary technologies relating to the production of cardiac stem cells for the treatment of heart disease.
For more information, please visitwww.vistagen.comand connect with VistaGen onTwitter,LinkedInandFacebook.
About NasdaqNasdaq (Nasdaq:NDAQ) is a leading provider of trading, clearing, exchange technology, listing, information and public company services across six continents. Through its diverse portfolio of solutions, Nasdaq enables clients to plan, optimize and execute their business vision with confidence, using proven technologies that provide transparency and insight for navigating today's global capital markets.As the creator of the world's first electronic stock market, its technology powers more than 85 marketplaces in 50 countries, and 1 in 10 of the world's securities transactions. Nasdaq is home to approximately 3,800 listed companies with a market value of $10.1 trillion and nearly 18,000 corporate clients. To learn more, visit: business.nasdaq.com.
-NDAQA-
Related Articles
Excerpt from:
VistaGen Therapeutics Inc. (Nasdaq: VTGN) to Ring The Nasdaq Stock Market Closing Bell - GlobeNewswire (press release)
TiGenix Announces Positive Topline Week-104 Data for Cx601 ADMIRE-CD Trial – P&T Community
By raymumme
TiGenix Announces Positive Topline Week-104 Data for Cx601 ADMIRE-CD Trial P&T Community Effective July 31, 2015, TiGenix acquired Coretherapix, whose lead cellular product candidate, AlloCSC-01, is currently in a Phase II clinical trial in Acute Myocardial Infarction (AMI). In addition, the second product candidate from the cardiac stem ... |
See the original post here:
TiGenix Announces Positive Topline Week-104 Data for Cx601 ADMIRE-CD Trial - P&T Community
Cardiac research nets Holly Mewhort prestigious heart association award – UCalgary News
By NEVAGiles23
Numerous people may say they want to grow up to be a heart surgeon, but very few actually achieve that goal. Holly Mewhort, MD, PhD, is one who has done so. And thats not the only thing Mewhort, who is part of the Libin Cardiovascular Institute of Albertas cardiac surgical residency program, has accomplished.
She has also excelled in basic and translational research. She recently received international recognition for her work in cardiac research, winning the Vivien Thomas Young Investigator Award from the American Heart Association, a prestige award given to early investigators who are focusing on fundamental and applied surgical research.
That research was done as part of her PhD program, which she completed in June 2016 under the supervision of Libin Institutes Paul Fedak, MD, PhD. Fedak is a cardiac surgeon and basic/translational researcher who directs the Marlene and Don Campbell Family Cardiac Research Laboratory at the Cumming School of Medicine.
Research shows biomaterial can trigger healing in damaged heart muscle
Mewhorts research investigates the use of biomaterial in regenerating and restoring heart tissue in patients who had previously suffered a heart attack. The material, CorMatrix-ECM, is a connective tissue matrix surgically applied to damaged heart tissue to trigger healing.Mewhort describes the material as providing the scaffolding that holds cells together and influences their behaviour and survival.
Her research in this area began four years ago in the lab and has had great success. In preclinical trials, the project has shown that this bio-material can restore function to damaged heart muscle by promoting the formation of new blood vessel networks a process called vasculogenesis.
The investigators have completed a pilot clinical trial, which saw the patch applied to the heart tissue of a handful of patients during coronary bypass surgery. The results havent been published yet, but the data looks promising.
Mewhort is thrilled to be part of a research project that has been successfully translated from bench to bedside. If it works at the clinical trial level, this could be a game-changer for patients who have suffered a heart attack, she says, noting until now, there hasnt been a way of restoring function to damaged heart tissue in those patients.
Cardiac surgery research program is 'cutting edge'
Its also exciting for Mewhort to win the same award her mentor, Dr. Paul Fedak, received 14 years ago. He was also a cardiac surgery trainee pursuing a PhD, investigating stem cell regeneration of heart tissue. The fact that two researchers connected with the University of Calgary earned the same international award is impressive, as competition is stiff. Past winners have studied at such institutions as the University of Toronto, Duke and Stanford.
Fedak, who studied at the University of Toronto, was recruited to come to Calgary about a decade ago and has since set up a cutting-edge cardiac surgery research program. Mewhort is the first PhD graduate of his laboratory. As her mentor, Fedak, who, besides being an academic researcher is a full-time clinical heart surgeon, is pleased to see the program turning out well-respected young academic surgical scientists.
He says Mewhorts award fulfils another career goal, explaining that when he won the Vivien Thomas Young Investigator Award his desire became to see one of his students do the same. For Fedak, the award signifies the coming-of-age of academic cardiac surgeon training in Calgary.
This shows us how far we have come with our program, he says.
Receiving her mentors praise is a big deal for Mewhort, as Fedak was one of the reasons she chose to pursue her surgery training and PhD in Calgary. Mewhorts future looks bright as she continues her residency in cardiac surgery on campus with the ultimate goal of having an active surgical and research career, much like her mentor.
Here is the original post:
Cardiac research nets Holly Mewhort prestigious heart association award - UCalgary News
Robert Clayton Robbins Top Choice for UA President – Arizona Public Media
By Sykes24Tracey
Robert Clayton Robbins, head of the Texas Medical Center, was named Tuesday as top choice for president of the University of Arizona.
The Board of Regents selected Robbins in Phoenix following interviews with him and one other candidate Monday.
Robbins will meet the campus community and the public at a forum Wednesday afternoon. The regents will vote next week formally to make him an offer, and contract negotiations will begin. A final vote on the contract is expected April 6, based on a timeline the regents released last week.
Robbins, who serves as president and chief executive officer at the Texas Medical Center, said at a press conference Tuesday he was eager to get on the road to Tucson. He said his top priority will be the UA's students.
"I look forward to meeting them, working with them, and helping them be prepared for this new world that were living in now," he said. "Its changing rapidly, and as the university family weve got to treat each one of them like our own children and help them be prepared for not just the four years they spend on campus, but the next 40 years of their life."
The announcement was delayed by more than an hour late Tuesday afternoon as members of the Board of Regents met privately to select their top candidate. Regent Bill Ridenour, who headed the search committee, said the delay was not a sign of disagreement.
"We just wanted to be very thorough," Ridenour said. "When you get nine people in a room that have differing thoughts, you want to make sure that you give those people every opportunity because its important, we think, that we be unanimous. So we are, and we are, and were excited."
Robbins is a cardiac surgeon who joined the Texas Medical Center as its president and CEO in 2012. In that time, he introduced five research initiatives centered on innovation, genomics, regenerative medicine, health policy and clinical research. The Texas Medical Center is the largest medical complex in the world, a press release said.
Dr. Robbins comprehensive experience as both a visionary leader and highly-respected physician, as well as his evident talent for advancing research, innovation, entrepreneurship and economic development will serve the University of Arizona and our state well, regents' President Eileen Klein said in a press release.
As a surgeon, Robbins has focused on acquired cardiac diseases with a special expertise in the surgical treatment of congestive heart failure and cardiothoracic transplantation. His research work includes the investigation of stem cells for cardiac regeneration.
The other finalist was Sethuraman Panch Panchanathan, executive vice president and chief research and innovation officer at Arizona State University.
Current UA President Ann Weaver Hart will step down after her successor is chosen. Hart will take a one-year sabbatical leave and return to the UA as a professor in the College of Education.
She became the university's first female president in 2012 and announced last year she would not seek renewal of her contract in 2018.
Read more:
Robert Clayton Robbins Top Choice for UA President - Arizona Public Media
Using Stem Cells to Predict Toxicity of Chemotherapy Drugs – ScienceBlog.com (blog)
By Dr. Matthew Watson
A team of scientists has developed a new safety index for a common group of chemotherapy drugs, by using a stem cell model to screen such therapies for their potential to damage patients hearts.
The study, published in Science Translational Medicine, was co-authored by Paul Burridge, PhD, assistant professor of Pharmacology.
Tyrosine kinase inhibitors (TKIs), a class of chemotherapy drugs, have become increasingly important in treating many types of cancer. But almost all TKIs are also associated with cardiovascular side effects ranging from arrhythmias to heart failure and there has not yet been an effective tool to predict this cardiotoxicity.
In the current study, the scientists demonstrated that human-induced pluripotent stem cells can be used to model how TKIs might affect the hearts of patients receiving chemotherapy.
To do so, the scientists took stem cells from both a control group and patients with cancer and reprogrammed them to become cardiomyocytes, or heart muscle cells. Using high-throughput screening, they then evaluated how the heart cells responded to treatment with 21 different FDA-approved TKIs, looking at factors like cell survival, signaling and alterations in their ability to beat properly.
With the stem-cell data, the scientists were able to create a cardiac safety index, which ranks the TKIs on their likelihood of inflicting heart damage. That index correlates with the toxicity that has been observed in patients clinically a validation that suggests the screening system might be a powerful tool in predicting toxicity before therapies are ever administered to patients.
Future research could establish even more specific predictions, by comparing the genomes of patients who might experience a certain drug side effect, such as atherosclerosis, with those who dont. Long-term, what my lab is interested in is taking a patients whole genome and, based on the work weve done in the past, being able to predict whether a patient will have an adverse drug event, said Burridge, also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. This is the whole idea of pharmacogenomics, or precision medicine: Everyone is going to have a different response to a drug, and that response good or bad is already encoded in all of us.
In the study, the scientists also discovered that administering insulin or insulin-like growth factor 1 alongside TKIs seemed to protect against some of the heart damage associated with the drugs. While its still early, this is the first step toward opening up a whole new field of identifying cardioprotectants to reduce the toxicity of these drugs, Burridge said.
The research was supported by the National Institutes of Health (NIH) grants K99/R00 HL121177, 14BGIA20480329, R01 HL132875, R01 HL130020, R01 HL128170, R01 HL123968, and R24 HL117756; the NIH Directors Pioneer Award; the American Heart Association Predoctoral Fellowship; the American Heart Association Beginning Grant-in-Aid; American Heart Association Grant-in-Aid; the American Heart Association Established Investigator Award; the National Science Foundation Graduate Research Fellowship; the Endowed Faculty Scholar Award of the Lucile Packard Foundation for Children and Child Health Research Institute at Stanford and Burroughs Wellcome Foundation Innovation in Regulatory Science.
See the original post here:
Using Stem Cells to Predict Toxicity of Chemotherapy Drugs - ScienceBlog.com (blog)
Stem Cell Therapy receives FDA Boost to enter the US Market – Labiotech.eu (blog)
By LizaAVILA
TiGenix has receivedpositive feedback from the FDA on an improved global phase III trial protocol for its lead candidateCx601 for Crohns disease. This is expected tospeed up US approval.
TiGenix is a Belgian companydevelopingstem cell therapies. The biotech is currently pushing its lead candidateCx601to the market for the treatment ofcomplex perianal fistulas in Crohns disease patients. Cx601 recently revealedpositive resultsin a European phase III study.
Following these results, the company submitted a number of technical adjustments for itspivotal phase III study for Biologics License Application (BLA) in the US, which were now approved by the FDA and are expected to acceleratethe process to US marketing authorization.
TiGenix is wellknown for its productChondroCellect, which was the first cell therapyto reach approval on the European market for the repair of knee cartilage.After the companyrecently withdrew its market authorization for this product, due to a lack of reimbursement, the biotech is focusing on its new leadCx601.
Thisproduct, currently awaiting EMA approval, consists ofallogeneic expanded adipose-derived stem cells (eASC), which are indicated for the treatment ofperianal fistulas in Crohns disease. The therapeuticeffects of eASCs are based on immunomodulatory abilities of these stem cells, which canrestore immune balance by suppressing a variety of immune cell subsets and inducing the generation of regulatory T cells.
Areas of the colon commonly affected duringCrohns disease
The current approval from the FDA will allow TiGenix to file the BLAbased on the efficacy and safety follow-up of patients at week 24, instead of week 52.The FDA has also agreed to accept fewer patients than originally planned in the study and endorsed a broader target population that will ultimately facilitate the recruitment process.
We believe that this revised protocol will allow us to file for approval one year earlier than we had originally plannedconcludedMaria Pascual, VP Regulatory Affairs & Corporate Quality of TiGenix
The current amendments will allow TiGenix to push its therapyto the US market even faster, which might pivotal for the company in light of its financial situation. After its shares had reached a low of22 cents back in 2013, the share price is currently still under 1. Withits low 34M IPO on Nasdaq in the end of last year, its market cap is stillonly at 191M. A low sum for a late stage clinical company.
As the EMAapproval forCx601 is expected soon, which will then be commercialized by Takeda, the company may actually be underestimated. The biotech recently started a new Phase Ib/IIa trial to testCx611 as a treatment for sepsis in patients with pneumonia.
Asecond platform consisting of transplanted allogeneic cardiac stem cells (AlloCSC)is currently in Phase II for acute myocardial infarction. It seems like TiGenix is definitely clinging toits position as one of the pioneers in stem cell-based therapies.
Images via shutterstock.com / CI Photos and CC 3.0 /RicHard-59
Continued here:
Stem Cell Therapy receives FDA Boost to enter the US Market - Labiotech.eu (blog)
Opinion/Commentary: Global stem cell therapy market to showcase growth – The Daily Progress
By JoanneRUSSELL25
LONDON Technavio analysts forecast the global stem cell therapy market to grow at a compound annual growth rate of close to 37 percent during the forecast period, according to their latest report.
The research study covers the present scenario and growth prospects of the global stem cell therapy market for 2017-2021. To determine the market size, the study considers revenue generated from allogenic and autogenic stem cell therapies.
The Americas are the largest regional segment of the global stem cell therapy market, responsible for generating over 56 percent of the total revenue (2016 figures). The region is expected to continue market dominance through the forecast period, driven by increasing demand for stem cell therapy products and investments into R&D.
Technavio analysts highlight the following factors as contributing to the growth of the global stem cell therapy market:
Increase in federal funding in stem cell therapy.
Sapna Jha, one of the lead research analysts at Technavio for medical imaging research, says, Many stem cell research institutes and small companies are involved in cutting-edge R&D and are yielding encouraging results. These institutions are witnessing an increased flow of investments from federal organizations, due to the realization of the importance of regenerative medicine.
The U.S. National Institutes of Health, a major funding government organization invested approximately USD 1.5 billion in stem cell research projects in 2016. Similarly, several state-level organizations such as California Institute for Regenerative Medicine has contributed USD 3 billion to stem cell research in 2014. Such funding will help various research institutes to discover and develop regenerative medicines, which will boost the global regenerative medicine market enormously.
Growing demand for personalized medicine.
The health care sector is creating a high demand for personalized medicine, which could offer game-changing opportunities for the vendors. These medicines offer treatments based on the individual characteristics, needs, and preferences, which will vastly improve the quality of health care. Individuals are increasingly banking their stem cells for future treatments. Research organizations are also extensively exploring ways to develop personalized treatments with stem cells, which could eventually erase the conventional medicine system and help in the effective treatment of various diseases such as diabetes and cancer.
Demand for development of effective drugs for cardiology and degenerative disorders.
There has been an increased demand to develop effective drugs for cardiology and degenerative disorders, for which there were no effective treatment plans before the advent of stem therapies. The discovery of possible cardiac stem cells uncovered new arenas to repair hearts injured due to acute myocardial infarction or coronary artery disease, says Sapna.
Researchers are studying and developing approximately 19 product candidates for the treatment of cardiac disorders, with eight of them in Phase III, and six in Phase II.
Technavio is a global technology research and advisory company. This report was made available through The Associated Press.
See the original post here:
Opinion/Commentary: Global stem cell therapy market to showcase growth - The Daily Progress
Heart Disease | Harvard Stem Cell Institute (HSCI)
By JoanneRUSSELL25
The Harvard Stem Cell Institute is developing new techniques to grow and transplant heart cells, replacing those lost to cardiovascular disease.
The greatest threat to the long-term health and well-being of people living with diabetes is cardiovascular disease. The diabetic population as a whole is two to four times more likely than non-diabetics to develop heart disease or suffer a stroke. Type 1 diabetes, which is most often diagnosed in childhood and adolescence, is particularly devastating, as one New England Journal of Medicine study associated it with a ten-fold increase in cardiovascular disease.
The human adult heart has about five billion heart cells, all pulsing as a coordinated orchestra with every heartbeat. These cells can be killed by high blood pressure, blood clots, heart attacks, and other byproducts of cardiovascular disease. The heart has an age-related block in its ability to make new heart cells, so that damaged cells are not replaced in the latter half of life, precisely when we need them the most. A typical patient with heart failure has lost over a billion heart cells.
Harvard Stem Cell Institute (HSCI) investigators are developing ways to make replacement heart cells and provide them with the right cues so that the new cells play as needed in the orchestra.
Both embryonic stem cells and induced pluripotent stem cells mature cells that are manipulated back to a stem cell state can be harnessed to create new heart cells. The difficulty is that the heart cells made with stem cells resemble the heart cells of an infant, rather than adult heart cells. To function in adult hearts, the new heart cells must mature and then be able to survive within the constantly beating environment of the heart.
The scientific community has generated the technology to make heart cells that are immature, but very few heart cells derived from stem cells integrate into the normal heart tissue as mature heart cells. At the HSCI, our researchers are focused on understanding how to take these new heart cells all the way to maturity and stability, so they can be used as an effective therapy.
HSCI scientists are also developing ways of using the bodys heart matrix the rich, intricate scaffold of the heart that serves as the permanent home for our heart cells to guide maturation and prolong the survival of heart cells derived from stem cells after implantation.
The heart matrix is like the sheet music for the heart orchestra. It tells the heart cells where to sit and how to function with their neighbors so that a heartbeat is in sync. The problem of redrawing these matrix-directed instructions from scratch once seemed too daunting to tackle.
By breaking down the hearts scaffold material into thousands of individual chemicals, HSCI researchers hope to rebuild the environments that allow immature heart cells to mature. Armed with this knowledge, it will be possible to construct real adult heart tissue in the laboratory, as well as realistic approaches to transplanting patient-specific heart cells into their damaged organs.
In addition to these ambitious projects, HSCI is pursuing interim objectives before reaching the ultimate goal of reconstructing the heart. For example, a recent study led to the identification of a blood circulating factor that declines with age but, when injected, can reverse age-related heart enlargement and accompanying heart failure. If this is successful in human studies, we will have identified a new therapeutic approach for the aging heart.
Original post:
Heart Disease | Harvard Stem Cell Institute (HSCI)
Stem cell therapy can help in treating diabetic heart disease – Business Standard
By LizaAVILA
Recent advancements in stem cells research have given hope for successfully treating diabetic heart disease (DHD), renowned New Zealand-based researcher in cardiovascular diseases Dr Rajesh Katare said on Tuesday.
DHD affected the muscular tissues of the heart leading to complications and it had been demonstrated that resident stem cells of myocardium can be stimulated to repair and replace e degenerated cardiac myocytes resulting in a novel therapeutic effect and ultimately cardiac regeneration, he said.
Katare, Director of Cardiovascular Research Division in the University of Otago, New Zealand, was delivering the keynote address at the continuing medical education programme on "Role of Micro-RNAs and stem cells in cardiac regeneration in diabetic heart disease" at the Karaikal campus of premier health institute JIPMER.
Presenting clinical evidences, Katare said stem cell therapy certainly presented a new hope for successfully treating DHD.
Jawaharlal Institute of Post Graduate Medical Education (JIPMER) Director Dr Subash Chandra Parija pointed out that it was the first such programme on the role of stem cells in cardiac regeneration in the whole of the country.
He said as diabetes was highly prevalent in the country, providing treatment for DHD had become a big challenge. Patients suffering from the condition have to undergo lifelong treatment and medications. "In this backdrop, advancements in stem cell therapy assume significance," he said. (REOPENS MES10)
Parija also said the government general hospital in
Karaikal being currently used by JIPMER for clinical teaching of students would have upgraded facilities.
He said a new building for the college would be constructed at a cost of Rs 497.10 crore soon.
The proposed up-gradation of the GH having 506 beds would help in imparting advanced clinical teaching and effective exposure of the medicos to various nuances of the diagnosis.
The Director also said JIPMER (Karaikal) had drawn up special post-graduate and fellowship programmes including on family medicine, tropical medicine, trauma care and cancer management.
Originally posted here:
Stem cell therapy can help in treating diabetic heart disease - Business Standard
Stem Cells and Aging | Life Code
By raymumme
Adult stem cell function declines with age leading to the decline in fitness
The potential therapeutic use of stem cells is a very hot topic these days. Most of the attention has focused on embryonic stem cells and induced Pluripotent Stem cells (iPS cells), which can form every tissue type in the body to regenerate failing organs. The problem is that detailed knowledge is lacking for how to stimulate the embryonic stem cells to form differentiated tissues (e.g. cells that form the heart, pancreas, muscle, and brain). Moreover, because embryonic stem cells are unlimited in their ability to form any type of tissue, the risk of cancer looms large over the therapeutic use of embryonic stem cells. For example, both embryonic and IPS stem cells can form tumors called teratomas when injected into immune-compromised mice. Enter the bodys adult stem cells, which have not generally been associated with cancer and have been used safely as therapeutics in many countries. The problem with adult stem cells is that it is difficult to get enough of them to be effective for most indications or target the harvested adult stem cells to the proper tissue. Moreover, there are scores of different types of adult stem cells in the body, so picking the best type of adult stem cell for a particular therapeutic can be challenging. Thus, adult stem cell therapeutics with all its potential to regenerate damaged organs and tissues is still a work in progress.
But what about the many populations of endogenous adult stem cells that everyone has embedded in every organ system of the body? All the organs and differing tissues of the body appear to have adult stem cells available for regenerating cells in case of injury or disease. It was recently discovered that even brain neurons and heart muscle cells (previously thought to be non-dividing and irreplaceable in adults) have their own reservoirs of adult stem cells for regeneration. Unfortunately, as we age most adult stem cell populations either decline in number and/or lose the ability to differentiate into functional tissue-specific cells. For example, cardiac muscle stem cells exist but old folks have only one half the number of cardiac stem cells found in young people. Thus, adult stem cells become more and more dysfunction with age, which progressively increases organ and tissue dysfunction with age.
There are many examples revealing the role of adult stem cells in aging. First, the outer surface of your skin continuously sloughs off dead cells, so that adult stem cells must continuously replenish the dying skin cells to maintain the skin as an effective protective barrier to the outside world. With age, there are progressively fewer functional skin stem cells, so cell turnover in the skin slows, leading to thinner, dryer skin that loses its elasticity and youthful beauty. Second, hair also thins and goes grey, as functional follicle stem cell decline and the adult stem cells generating hair color also decline. Third, the differing adult stem cells that maintain the tissues composing skeletal muscle, pancreas, heart, bone, liver, kidney, and the immune system lose functional capacity, raising the potential for decline in tissue function or outright failure with age. As a final example, the five senses of sight, hearing, smell, taste, and touch slowly wane with age, as the declining stem cell populations responsible for maintaining these functions are unable to fully replenish the sensory neurons after injury and random cell death.
If your own adult stem cells are a key factor in aging and disease, then one novel way to slow aging and disease is to stimulate your own adult stem cells to maintain their proper numbers and functional capacity to differentiate into the various tissues as needed for repair and regeneration. This makes sense, because in most, if not all, organs of the body, old cells are continually being replaced by new cells coming from the adult stem cell populations. If stem cells are not producing enough new cells, then organs slowly decline in function as you age. Thus, stimulating your own stem cells can be a winning strategy to stave off many of the disorders associated with aging.
In practice, however, stimulating adult stem cell populations in the body is not a simple task. If the proliferation of adult stem cells is over stimulated, then one may get overgrowth of tissues or a potential tumor. Alternatively, one may stimulate the stem cells to proliferate in a balanced and regulated way, but the stem cells lose functionality and cannot differentiate into the desired specialized tissues to replace senescent cells. These twin problems promoting over stimulation or dysfunctional stem cells put real limits on any proposed therapeutic for stimulating stem cells. For example, most current treatments to stimulate immunity or stem cells (nave T cells) rely on complex carbohydrates from mushrooms or microorganisms to provide antigenic material that can stimulate immunity. This will activate the immune system stem cells to make more differentiated non-stem memory T cells directed against the antigenic material, but it does nothing to stimulate more immune stem cells (nave T cells). Indeed, chronic use of such stem cell enhancers may actually lead to stem cell depletion, as more adult stem cells are exhausted from the requirement to respond to the constant presence of the polysaccharide antigen. Indeed, one theory of how the HIV virus causes a defective immune system is that it exhausts the supply of nave T cells by the repeated attacks of the mutating HIV virus.
Stem Cell 100TM is a nutraceutical supplement that improves the function of your existing stem cells rather than over stimulate stem cells to differentiate or divide. By promoting the stability and vitality of adult stem cells they have the capacity to divide when the body signals a need for more stem cells and differentiated cells. When an organ or tissue is damaged, it will send out natural signals that new cells are needed to replace old or damaged cells. Stem Cell 100TM allows the adult stem cells to respond to the damage signal by provided new differentiated cells to replace the old damaged cells and also make more adult stem cells to keep up the stem cell population. Two other compounds in Stem Cell 100TM provide further natural support for stem cells.
(Note that not everyone will experience the same effects, as conditions vary among individuals. The general expectation is that for most health measurements that are in the Normal Range for your age, Stem Cell 100TM will promote readings that you had when some 20 years younger.)
The statements above have not been reviewed by the FDA. Stem Cell 100TM is not meant as a preventive or treatment for any disease.
See the original post here:
Stem Cells and Aging | Life Code
Stem cell therapy can help treat diabetic heart disease – The … – Economic Times
By LizaAVILA
KARAIKAL: Recent advancements in stem cells research have given hope for successfully treating diabetic heart disease (DHD), renowned New Zealand-based researcher in cardiovascular diseases Dr Rajesh Katare said today.
DHD affected the muscular tissues of the heart leading to complications and it had been demonstrated that resident stem cells of myocardium can be stimulated to repair and replace e degenerated cardiac myocytes resulting in a novel therapeutic effect and ultimately cardiac regeneration, he said.
Katare, Director of Cardiovascular Research Division in the University of Otago, New Zealand, was delivering the keynote address at the continuing medical education programme on "Role of Micro-RNAs and stem cells in cardiac regeneration in diabetic heart disease" at the Karaikal campus of premier health institute JIPMER.
Presenting clinical evidences, Katare said stem cell therapy certainly presented a new hope for successfully treating DHD.
Jawaharlal Institute of Post Graduate Medical Education (JIPMER) Director Dr Subash Chandra Parija pointed out that it was the first such programme on the role of stem cells in cardiac regeneration in the whole of the country.
He said as diabetes was highly prevalent in the country, providing treatment for DHD had become a big challenge. Patients suffering from the condition have to undergo lifelong treatment and medications. "In this backdrop, advancements in stem cell therapy assume significance," he said.
Go here to read the rest:
Stem cell therapy can help treat diabetic heart disease - The ... - Economic Times
StemBioSys Lands Experimental UT Tech That Finds Young Stem Cells – Xconomy
By Sykes24Tracey
Xconomy Texas
San Antonio StemBioSys, the life sciences company with a system for growing stem cells, has licensed an experimental technology from University of Texas Health San Antonio that may help identify healthy young adult stem cells among large pools of other cells.
Theres plenty of research examining how to possibly use adult stem cells as treatments for medical conditions, ranging from cardiac disease to metabolic disorders, but current uses are rather limited to therapies like bone-marrow transplants for blood disorders, especially in children. Treatments that use patients own stem cells may be safer than using stem cells from someone else because they might reduce the potential for an immune response, according to StemBioSys CEO Bob Hutchens. Thats still theoretical, he says.
Finding large quantities of usable adult stem cells is difficult, though. StemBioSys believes its new technology can potentially identify a few thousand high-quality, young stem cells from a sample of tens of thousands of cells taken from a patient, Hutchens sayspotentially being a key word.
The research is quite earlythe technology has only been studied in animal models and in vitro, and StemBioSys is in the process of applying for federal grants to take the research into animal trials. If StemBioSys new intellectual property can successfully isolate the stem cells, Hutchens says they could grow more of them with StemBioSys core product.
StemBioSys sells a so-called extracellular matrix product made of proteins that provide a hospitable environment for stem cells, helping them divide and produce more stem cells.
Whats intriguing to us is that its a really interesting application of our technology, Hutchens says. You take this combination of identifying this very small population of young healthy cells in elderly people, and use our technology to expand it.
If the company can indeed find the young stem cells of a single patient and replicate them, it would give researchers and physician an accessible pool of the cells that theyd want for potential stem cell transplants and other treatments, Hutchens says.
Terms of the deal werent disclosed. StemBioSys, which was founded based on other University of Texas System research, acquired a portfolio of issued and pending patents. Famed MIT researcher and Xconomist Robert Langer is on the companys board of directors.
Again, theres plenty to prove out with this early stage research, so it will take time before any potential commercialization comes to fruition. Travis Block, the researcher who helpeddevelopthe technology while earning his PhD. last year at the University of Texas Health Science Center at San Antonio, will help shepherd the project along and other regenerative medicine work as StemBioSyss senior scientist.
David Holley is Xconomy's national correspondent based in Austin, TX. You can reach him at dholley@xconomy.com
See more here:
StemBioSys Lands Experimental UT Tech That Finds Young Stem Cells - Xconomy
TiGenix to present at Cowen’s 37th Annual Health Care Conference in Boston – EconoTimes
By LizaAVILA
Wednesday, March 1, 2017 6:01 AM UTC
PRESS RELEASE
TiGenix to present at Cowen's 37thAnnual Health Care Conference in Boston
Leuven (BELGIUM) - 1st March, 2017, 07:00h CET - TiGenix NV (Euronext Brussels and Nasdaq: TIG), an advanced biopharmaceutical company focused on developing and commercializing novel therapeutics from its proprietary platforms of allogeneic expanded stem cells, today announced that Eduardo Bravo, CEO of TiGenix, will be presenting at the 37th Annual Cowen and Company's Health Care Conference in Boston (USA) at The Boston Marriott Copley Place on Monday, March 6 at 3:20-3:50PM (EST) in Regis, 3rd Floor (breakout at 4:00 PM-04:30PM (EST) at Boston University, 3rd Floor).
The presentation will be webcast live and can be accessed on the day of the event at this link. A replay of the webcast will be available on the Company's website for 30 days following the presentation. To ensure timely connection, it is recommended that users register at least 10 minutes prior to the scheduled webcast.
The TiGenix management team will be available for one-to-one meetings from Monday, March 6th to Wednesday, March 8th. Please contact Investor Relations at Investor@tigenix.com for a meeting request.
For more information:
Claudia D'Augusta Chief Financial Officer T: +34 91 804 92 64 claudia.daugusta@tigenix.com
About TiGenix
TiGenix NV (Euronext Brussels: TIG) is an advanced biopharmaceutical company focused on developing and commercializing novel therapeutics from its proprietary platforms of allogeneic, or donor-derived, expanded stem cells. Our lead product candidate from the adipose-derived stem cell technology platform is Cx601, which is in registration with the European Medicines Agency for the treatment of complex perianal fistulas in Crohn's disease patients. Our adipose-derived stem cell product candidate Cx611 has completed a Phase I sepsis challenge trial and a Phase I/II trial in rheumatoid arthritis. Effective July 31, 2015, TiGenix acquired Coretherapix, whose lead cellular product candidate, AlloCSC-01, is currently in a Phase II clinical trial in Acute Myocardial Infarction (AMI). In addition, the second product candidate from the cardiac stem cell-based platform acquired from Coretherapix, AlloCSC-02, is being developed in a chronic indication. On July 4, 2016, TiGenix entered into a licensing agreement with Takeda, a large pharmaceutical company active in gastroenterology, under which Takeda acquired the exclusive right to commercialize Cx601 for complex perianal fistulas outside the United States. TiGenix is headquartered in Leuven (Belgium) and has operations in Madrid (Spain).
About Cx601
Cx601 is a suspension of allogeneic expanded adipose-derived stem cells (eASC) locally injected. Cx601 is an investigational agent being developed for the treatment of complex perianal fistulas in Crohn's disease patients with inadequate response to at least one conventional or biologic therapy including antibiotics, immunosuppressants, or anti-TNF agents. Crohn's disease is a chronic inflammatory disease of the intestine and patients can suffer from complex perianal fistulas for which there is currently no effective treatment. In 2009, the European Commission granted Cx601 orphan designation for the treatment of anal fistulas, recognizing the debilitating nature of the disease and the lack of treatment options. Cx601 has met the primary end-point in the Phase III ADMIRE-CD study in Crohn's disease patients with complex perianal fistula, a randomized, double-blind, placebo-controlled trial run in Europe and Israel and designed to comply with the requirements laid down by the EMA. 'Madrid Network' issued a soft loan to help finance this Phase III study, which was funded by the Secretary of State for Research, Development and Innovation (Ministry of Economy and Competitiveness) within the framework of the INNTEGRA plan. The study's primary endpoint was combined remission, defined as clinical assessment at week 24 of closure of all treated external openings draining at baseline despite gentle finger compression, and absence of collections >2cm confirmed by MRI. In the ITT population (n=212), Cx601 achieved statistically significant superiority (p=0.024) on the primary endpoint with 50% combined remission at week 24 compared to 34% in the placebo arm. Efficacy results were robust and consistent across all statistical populations. Treatment emergent adverse events (non-serious and serious) and discontinuations due to adverse events were comparable between Cx601 and placebo arms. The 24-weeks results have been published by The Lancet, one of the most highly regarded and well known medical journals in the world. The Phase III study has completed a follow-up analysis at 52 weeks confirming its sustained efficacy and safety profile. Top line follow-up data showed that in the ITT population Cx601 achieved statistical superiority (p=0.012) with 54% combined remission at week 52 compared to 37% in the placebo arm. The 52-week data also showed a higher rate of sustained closure in those patients treated with Cx601 and in combined remission at week 24 (75.0%) compared to patients in the placebo group (55.9%). Based on the positive 24-weeks Phase III study results, TiGenix has submitted a Marketing Authorization Application to the EMA in early 2016. TiGenix is preparing to develop Cx601 in the U.S. after having reached an agreement with the FDA through a special protocol assessment procedure (SPA) in 2015. On July 4, 2016 TiGenix entered into a licensing agreement with Takeda, a pharmaceutical company leader in gastroenterology, whereby Takeda acquired an exclusive right to commercialize Cx601 for complex perianal fistulas in Crohn's patients outside of the U.S.
Forward-looking information
This press release may contain forward-looking statements and estimates with respect to the anticipated future performance of TiGenix and the market in which it operates. Certain of these statements, forecasts and estimates can be recognised by the use of words such as, without limitation, "believes", "anticipates", "expects", "intends", "plans", "seeks", "estimates", "may", "will" and "continue" and similar expressions. They include all matters that are not historical facts. Such statements, forecasts and estimates are based on various assumptions and assessments of known and unknown risks, uncertainties and other factors, which were deemed reasonable when made but may or may not prove to be correct. Actual events are difficult to predict and may depend upon factors that are beyond the Company's control. Therefore, actual results, the financial condition, performance or achievements of TiGenix, or industry results, may turn out to be materially different from any future results, performance or achievements expressed or implied by such statements, forecasts and estimates. Given these uncertainties, no representations are made as to the accuracy or fairness of such forward-looking statements, forecasts and estimates. Furthermore, forward-looking statements, forecasts and estimates only speak as of the date of the publication of this press release. TiGenix disclaims any obligation to update any such forward-looking statement, forecast or estimates to reflect any change in the Company's expectations with regard thereto, or any change in events, conditions or circumstances on which any such statement, forecast or estimate is based, except to the extent required by Belgian law.
Human Life Could Be Extended Indefinitely, Study Suggests
Goosebumps, tears and tenderness: what it means to be moved
Are over-the-counter painkillers a waste of money?
Does an anomaly in the Earth's magnetic field portend a coming pole reversal?
Immunotherapy: Training the body to fight cancer
Do vegetarians live longer? Probably, but not because they're vegetarian
Could a contraceptive app be as good as the pill?
Some scientific explanations for alien abduction that aren't so out of this world
Society actually does want policies that benefit future generations
Six cosmic catastrophes that could wipe out life on Earth
Big Pharma Starts Using Cannabis For Making Drugs In Earnest
Do you need to worry if your baby has a flat head?
View post:
TiGenix to present at Cowen's 37th Annual Health Care Conference in Boston - EconoTimes
Johns Hopkins Medicine, Maryland Stem Cell Research Fund and … – Business Wire (press release)
By daniellenierenberg
SAN CARLOS, Calif. & BALTIMORE--(BUSINESS WIRE)--Johns Hopkins Medicine, the Maryland Stem Cell Research Fund (MSCRF) and BioCardia, Inc. (OTC:BCDA) today announced that the first patient has been treated in the pivotal Phase III CardiAMP clinical trial of a cell-based therapy for the treatment of ischemic heart failure that develops after a heart attack. The first patient was treated at Johns Hopkins Hospital by a team led by Peter Johnston, MD, a faculty member in the Department of Medicine and Division of Cardiology, and principal investigator of the trial at Johns Hopkins.
The investigational CardiAMP therapy is designed to deliver a high dose of a patients own bone marrow cells directly to the point of cardiac dysfunction, potentially stimulating the bodys natural healing mechanism after a heart attack.
The patient experience with CardiAMP therapy begins with a pre-procedural cell potency screening test. If a patient qualifies for therapy, they are scheduled for a bone marrow aspiration. A point of care cell processing platform is then utilized to concentrate the autologous bone marrow cells, which are subsequently delivered in a minimally-invasive procedure directly to the damaged regions in a patients heart.
This cell-based therapy offers great potential for heart failure patients, said Carl Pepine, MD, professor and former chief of cardiovascular medicine at the University of Florida, Gainesville and national co-principal investigator of the CardiAMP trial. We look forward to validating the impact of the therapy on patients quality of life and functional capacity in this important study.
In addition to Dr. Johnston, the CardiAMP research team at Johns Hopkins includes Gary Gerstenblith, MD, Jeffrey Brinker, MD, Ivan Borrello, MD, Judi Willhide, Katherine Laws, Audrey Dudek, Michele Fisher and John Texter, as well as the nurses and technicians of the Johns Hopkins Cardiovascular Interventional Laboratory.
Funding the clinical trial of this cell therapy, which could be the first cardiac cell therapy approved in the United States, is an important step towards treatments, said Dan Gincel, PhD., executive director of the MSCRF at TEDCO. Through our clinical program, we are advancing cures and improving healthcare in the State of Maryland.
The CardiAMP Heart Failure Trial is a phase III, multi-center, randomized, double-blinded, sham-controlled study of up to 260 patients at up to 40 centers nationwide, which includes an optional 10-patient roll-in cohort. The primary endpoint for the trial is a significant improvement in Six Minute Walk distance at 12 months post-treatment. Study subjects must be diagnosed with New York Heart Association (NYHA) Class II or III heart failure as a result of a previous heart attack. The national co-principal investigators are Dr. Pepine and Amish Raval, MD, of the University of Wisconsin.
For information about eligibility or enrollment in the trial, please visit http://www.clinicaltrials.gov or ask your cardiologist.
About BioCardia BioCardia, Inc., headquartered in San Carlos, CA, is developing regenerative biologic therapies to treat cardiovascular disease. CardiAMP and CardiALLO cell therapies are the companys biotherapeutic product candidates in clinical development. For more information, visit http://www.BioCardia.com.
About Johns Hopkins Medicine Johns Hopkins Medicine (JHM), headquartered in Baltimore, Maryland, is one of the leading health care systems in the United States. Johns Hopkins Medicine unites physicians and scientists of the Johns Hopkins University School of Medicine with the organizations, health professionals and facilities of The Johns Hopkins Hospital and Health System. For more information, visit http://www.hopkinsmedicine.org.
About Maryland Stem Cell Research Fund The Maryland Stem Cell Research Act of 2006was established by the Governor and the Maryland General Assembly during the 2006 legislative session and created the Maryland Stem Cell Research Fund. This fund is continued through an appropriation in the Governor's annual budget. The purpose of the Fund is to promote state-funded stem cell research and cures through grants and loans to public and private entities in the State. For more information, visit http://www.MSCRF.org.
Forward Looking Statements This press release contains forward-looking statements as that term is defined under the Private Securities Litigation Reform Act of 1995. Such forward-looking statements include, among other things, references to the enrollment of our Phase 3 trial, commercialization and efficacy of our products and therapies, the product development timelines of our competitors. Actual results could differ from those projected in any forward-looking statements due to numerous factors. Such factors include, among others, the inherent uncertainties associated with developing new products or technologies, unexpected expenditures, the ability to raise the additional funding needed to continue to pursue BioCardias business and product development plans, competition in the industry in which BioCardia operates and overall market conditions, and whether the combined funds will support BioCardias operations and enable BioCardia to advance its pivotal Phase 3 CardiAMP cell therapy program. These forward-looking statements are made as of the date of this press release, and BioCardia assumes no obligation to update the forward-looking statements.
The rest is here:
Johns Hopkins Medicine, Maryland Stem Cell Research Fund and ... - Business Wire (press release)
Stem cell therapy can help in treating diabetic heart disease – India.com
By LizaAVILA
Karaikal, Feb 28 (PTI) Recent advancements in stem cells research have given hope for successfully treating diabetic heart disease (DHD), renowned New Zealand-based researcher in cardiovascular diseases Dr Rajesh Katare said today.
DHD affected the muscular tissues of the heart leading to complications and it had been demonstrated that resident stem cells of myocardium can be stimulated to repair and replace e degenerated cardiac myocytes resulting in a novel therapeutic effect and ultimately cardiac regeneration, he said.
Katare, Director of Cardiovascular Research Division in the University of Otago, New Zealand, was delivering the keynote address at the continuing medical education programme on Role of Micro-RNAs and stem cells in cardiac regeneration in diabetic heart disease at the Karaikal campus of premier health institute JIPMER.
Presenting clinical evidences, Katare said stem cell therapy certainly presented a new hope for successfully treating DHD.
Jawaharlal Institute of Post Graduate Medical Education (JIPMER) Director Dr Subash Chandra Parija pointed out that it was the first such programme on the role of stem cells in cardiac regeneration in the whole of the country.
He said as diabetes was highly prevalent in the country, providing treatment for DHD had become a big challenge.
Patients suffering from the condition have to undergo lifelong treatment and medications. In this backdrop, advancements in stem cell therapy assume significance, he said.
This is published unedited from the PTI feed.
Antodaya, Humsafar Express to be launched today; Suresh Prabhu to unveil new catering policy for railways today
Uttar Pradesh Assembly elections 2017 LIVE: 57.36% voter turnout recorded till 5 PM in the fifth phase
Oscars 2017: Late Bollywood veteran Om Puri remembered at 89th Academy Awards!
Oscars 2017: Moonlight star Mahershala Ali is now best 'Actor in a Supporting Role'
Oscar Awards 2017 Live: When is Oscars 2017? Where to watch 89th Academy Awards LIVE Streaming & online telecast in India?
View original post here:
Stem cell therapy can help in treating diabetic heart disease - India.com


 March 16th, 2017
March 16th, 2017