RespireRx Pharmaceuticals Inc. Announces Appointment of Dr. James Cook and Dr. Jeffrey Witkin as Research Fellows
By Dr. Matthew Watson

See the original post:
RespireRx Pharmaceuticals Inc. Announces Appointment of Dr. James Cook and Dr. Jeffrey Witkin as Research Fellows
Rafael Pharmaceuticals Crosses Enrollment of 100th Patient in Pivotal Phase 3 Trial (ARMADA 2000) of CPI-613® (Devimistat) for Relapsed or Refractory…
By Dr. Matthew Watson
Company continues meeting enrollment milestones across clinical trials amid the COVID-19 pandemic, putting patient safety first Company continues meeting enrollment milestones across clinical trials amid the COVID-19 pandemic, putting patient safety first
Read the rest here:
Rafael Pharmaceuticals Crosses Enrollment of 100th Patient in Pivotal Phase 3 Trial (ARMADA 2000) of CPI-613® (Devimistat) for Relapsed or Refractory...
BioStem Technologies, Inc. Announces Launch of AEON™
By Dr. Matthew Watson
Pompano Beach, Fl., Oct. 27, 2020 (GLOBE NEWSWIRE) -- BioStem Technologies, Inc. (OTC PINK: BSEM) ("BioStem" or the "Company"), a leading life sciences company specializing in perinatal tissue allografts for use in regenerative therapies, today announced the launch of AEON™, the 6th and newest addition to the Company’s perinatal tissue allograft platform.
Go here to see the original:
BioStem Technologies, Inc. Announces Launch of AEON™
Akari Therapeutics to Present Phase II Data from Bullous Pemphigoid Trial at European Academy of Dermatology and Venereology (EADV) Virtual Congress…
By Dr. Matthew Watson
NEW YORK and LONDON, Oct. 27, 2020 (GLOBE NEWSWIRE) -- Akari Therapeutics, Plc (Nasdaq: AKTX), a biopharmaceutical company focused on innovative therapeutics to treat orphan autoimmune and inflammatory diseases where the complement and/or leukotriene systems are implicated, today announces that it will be presenting a poster during the 29th European Academy of Dermatology and Venereology (EADV) Congress being held virtually October 28 – November 1, 2020. The presentation will be posted onto Akari’s website.
Go here to read the rest:
Akari Therapeutics to Present Phase II Data from Bullous Pemphigoid Trial at European Academy of Dermatology and Venereology (EADV) Virtual Congress...
CohBar to Participate at ROTH Capital COVID-19 Therapeutics in Development Event
By Dr. Matthew Watson
MENLO PARK, Calif., Oct. 27, 2020 (GLOBE NEWSWIRE) -- CohBar, Inc. (NASDAQ: CWBR), a clinical stage biotechnology company developing mitochondria based therapeutics to treat chronic diseases and extend healthy lifespan, announced today that its Chief Executive Officer, Steven Engle, and Chief Scientific Officer, Dr. Kenneth Cundy, will participate on the panel titled “Direct Antivirals and Other Agents Against SARS-CoV2 Virus” at the ROTH Capital COVID-19 Therapeutics in Development Event, being held virtually on October 28, 2020 at 7:30am PT. A live webcast of the presentation will be available for attendees who register at https://roth.zoom.us/webinar/register/WN_FF_LgnOeQmm7VsRye97DtQ.
Excerpt from:
CohBar to Participate at ROTH Capital COVID-19 Therapeutics in Development Event
Novavax Provides Phase 3 COVID-19 Vaccine Clinical Development Update
By Dr. Matthew Watson
GAITHERSBURG, Md., Oct. 27, 2020 (GLOBE NEWSWIRE) -- Novavax, Inc. (Nasdaq: NVAX), a late-stage biotechnology company developing next-generation vaccines for serious infectious diseases, announced updates on its Phase 3 clinical development program of NVX-CoV2373, its COVID-19 vaccine candidate. NVX?CoV2373 is a stable, prefusion protein made using Novavax’ nanoparticle technology and includes Novavax’ proprietary Matrix?M™ adjuvant. The Company also announced that it will present data from its ongoing Phase 1/2 clinical trial, including new Phase 2 reactogenicity data, on Friday, October 30 during the United States (U.S.) Center for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices’ (ACIP) meeting.
Read this article:
Novavax Provides Phase 3 COVID-19 Vaccine Clinical Development Update
Galapagos’ R&D Roundtable showcases Toledo program
By Dr. Matthew Watson
Mechelen, Belgium; 27 October 2020, 16.15 CET – Galapagos NV (Euronext & NASDAQ: GLPG) unveils the Toledo target family as a series of salt-inducible kinase inhibitors. Toledo exhibits a dual mode of action characterized by enhanced transcription of anti-inflammatory cytokines and inhibited transcription of pro-inflammatory cytokines. Today, Galapagos also presents new preclinical and healthy volunteer data, and details its broad program to discover and develop multiple series of Toledo compounds with different selectivity profiles, aimed at treating a broad range of autoimmune conditions with important unmet medical need.
Follow this link:
Galapagos’ R&D Roundtable showcases Toledo program
Indus Holdings, Inc. to Report Third Quarter 2020 Financial and Operational Results
By Dr. Matthew Watson
California cannabis leader to host conference call on November 10, 2020 California cannabis leader to host conference call on November 10, 2020
Link:
Indus Holdings, Inc. to Report Third Quarter 2020 Financial and Operational Results
Tiziana Life Sciences Announces Timetable of Demerger of Accustem (for holders of Tiziana ADRs)
By Dr. Matthew Watson
NEW YORK and LONDON, Oct. 27, 2020 (GLOBE NEWSWIRE) -- Tiziana Life Sciences plc (Nasdaq: TLSA / AIM: TILS) ("Tiziana" or the "Company"), a biotechnology company focused on innovative therapeutics for oncology, inflammation and infectious diseases, today announces that it is confirming the timetable for the Accustem Sciences Limited ("Accustem") demerger (the "Demerger") for holders of the Company’s American Depositary Receipts (“ADRs”).
Read more:
Tiziana Life Sciences Announces Timetable of Demerger of Accustem (for holders of Tiziana ADRs)
Sanofi: Information concerning the total number of voting rights and shares – September 2020
By Dr. Matthew Watson
Information concerning the total number of voting rights and shares, provided pursuant to article L. 233-8 II of the Code de commerce (the French Commercial Code) and article 223-16 of the Règlement général de l’Autorité des Marchés Financiers (Regulation of the French stock market authority)
More here:
Sanofi: Information concerning the total number of voting rights and shares - September 2020
Galera Therapeutics Announces Interim Data from Pilot Phase 1/2 Trial of GC4419 in Combination with Stereotactic Body Radiation Therapy Showed…
By Dr. Matthew Watson
Initial results from pilot Phase 1/2 clinical trial in patients with locally advanced pancreatic cancer presented during virtual ASTRO Annual Meeting
Read the rest here:
Galera Therapeutics Announces Interim Data from Pilot Phase 1/2 Trial of GC4419 in Combination with Stereotactic Body Radiation Therapy Showed...
Lumos Pharma to Report Third Quarter 2020 Financial Results and Host a Conference Call on November 10, 2020
By Dr. Matthew Watson
AUSTIN, Texas, Oct. 27, 2020 (GLOBE NEWSWIRE) -- Lumos Pharma, Inc. (NASDAQ:LUMO), a clinical-stage biopharmaceutical company focused on therapeutics for rare diseases, today announced it will report its third quarter 2020 financial results after market close on Tuesday, November 10, 2020. The company will host a conference call and webcast at 4:30 PM ET that day to discuss its third quarter financial results and provide an update on corporate activities. There will also be a question and answer session following the prepared remarks.
Vir Biotechnology to Provide Corporate Update and Report Third Quarter 2020 Financial Results on November 10, 2020
By Dr. Matthew Watson
SAN FRANCISCO, Oct. 27, 2020 (GLOBE NEWSWIRE) -- Vir Biotechnology, Inc. (Nasdaq: VIR), a clinical-stage immunology company focused on treating and preventing serious infectious diseases, today announced that it will provide a corporate update and report financial results for the third quarter ended September 30, 2020 on Tuesday, November 10, 2020.
Opiant Pharmaceuticals to Report Third Quarter 2020 Financial Results and Host Conference Call and Webcast on November 12, 2020
By Dr. Matthew Watson
SANTA MONICA, Calif., Oct. 27, 2020 (GLOBE NEWSWIRE) -- Opiant Pharmaceuticals, Inc. (“Opiant”) (NASDAQ: OPNT), a specialty pharmaceutical company developing medicines for addictions and drug overdose, today announced it will report its third quarter 2020 financial results after the financial markets close on Thursday November 12, 2020.
Read the original:
Opiant Pharmaceuticals to Report Third Quarter 2020 Financial Results and Host Conference Call and Webcast on November 12, 2020
Aptose to Release Third Quarter Ended September 30, 2020 Financial Results and Hold Conference Call on November 10, 2020
By Dr. Matthew Watson
SAN DIEGO and TORONTO, Oct. 27, 2020 (GLOBE NEWSWIRE) -- Aptose Biosciences Inc. (Nasdaq: APTO; TSX: APS), a clinical-stage company developing highly differentiated therapeutics that target the underlying mechanisms of cancer, will release its financial results for the quarter ended September 30, 2020 on Tuesday, November 10, 2020, after the close of the market.
Read the original post:
Aptose to Release Third Quarter Ended September 30, 2020 Financial Results and Hold Conference Call on November 10, 2020
Repligen Corporation Announces Agreement to Acquire Bioprocess Systems Innovator ARTeSYN Biosolutions and Completes Acquisition of Non-Metallic…
By Dr. Matthew Watson
WALTHAM, Mass., Oct. 27, 2020 (GLOBE NEWSWIRE) -- Repligen Corporation (NASDAQ:RGEN), a life sciences company focused on bioprocessing technology leadership, today announced that it has entered into a definitive agreement to acquire privately-held ARTeSYN Biosolutions (“ARTeSYN”) for approximately $200 million, comprised of approximately $130 million in cash and approximately $70 million in Repligen common stock. ARTeSYN Biosolutions is projected to generate approximately $30 million in revenue (pro forma) in 2020, led by the success of its single-use chromatography and filtration systems which are considered the gold standards in downstream bioprocessing due to their performance, automation and low hold-up volumes. The proposed acquisition of ARTeSYN, combined with the recent acquisitions of Engineered Molding Technologies (“EMT”) and Non-Metallic Solutions (“NMS”) further establishes Repligen as a premier player in single-use systems and associated integrated flow path assemblies.
Continue reading here:
Repligen Corporation Announces Agreement to Acquire Bioprocess Systems Innovator ARTeSYN Biosolutions and Completes Acquisition of Non-Metallic...
Transactions in connection with and conclusion of share buyback program
By Dr. Matthew Watson
Company announcement no. 42 - 20 27 October 2020
Read more:
Transactions in connection with and conclusion of share buyback program
Supernus to Host Third Quarter 2020 Financial Results Conference Call
By Dr. Matthew Watson
ROCKVILLE, Md., Oct. 27, 2020 (GLOBE NEWSWIRE) -- Supernus Pharmaceuticals, Inc. (Nasdaq: SUPN), a pharmaceutical company focused on developing and commercializing products for the treatment of central nervous system (CNS) diseases, today announced that the Company expects to report business results for the third quarter of 2020 after 5:00 p.m. ET on Tuesday, November 3, 2020.
Link:
Supernus to Host Third Quarter 2020 Financial Results Conference Call
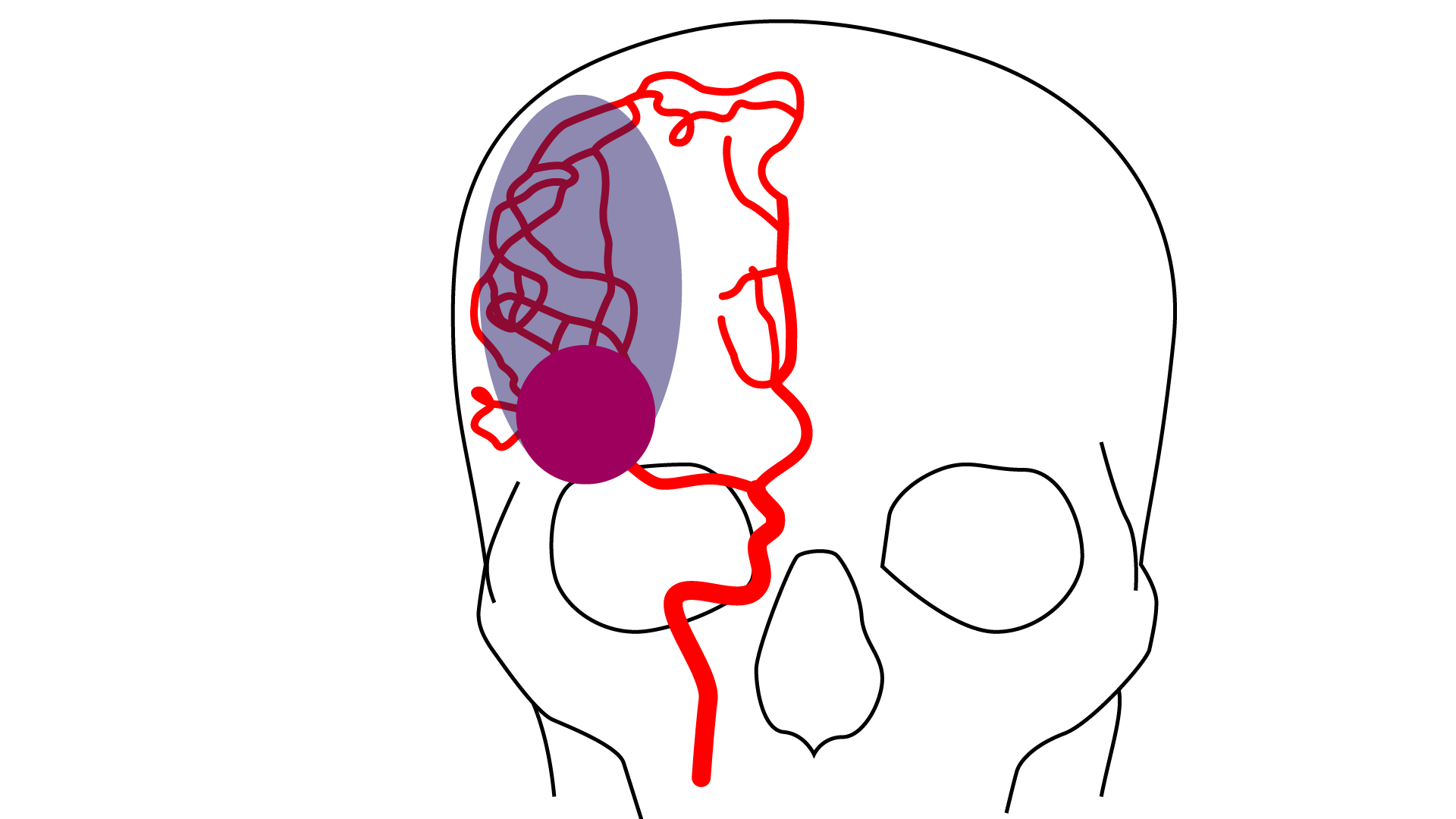
CLINUVEL to Trial Innovative Drug in Stroke
By Dr. Matthew Watson
To Read More: CLINUVEL to Trial Innovative Drug in StrokeStem cell treatment after spinal cord injury: The next …
By daniellenierenberg
June 27, 2020
Following promising phase 1 testing, Mayo Clinic is launching phase 2 of a randomized clinical trial of stem cell treatment for patients with severe spinal cord injury. The clinical trial, known as CELLTOP, involves intrathecal injections of autologous adipose-derived stem cells.
"The field of spinal cord injury has seen advances in recent years, but nothing in the way of a significant paradigm shift. We currently rely on supportive care. Our hope is to alter the course of care for these patients in ways that improve their lives," says Mohamad Bydon, M.D., a neurosurgeon at Mayo Clinic in Rochester, Minnesota.
The first participant in the phase 1 trial was a superresponder who, after stem cell therapy, saw significant improvements in the function of his upper and lower extremities.
"Not every patient who receives stem cell treatment is going to be a superresponder. Among the 10 participants in our phase 1 study, we had some nonresponders and moderate responders," Dr. Bydon says. "One objective in our future studies is to delineate the optimal treatment protocols and understand why patients respond differently."
In CELLTOP phase 2, 40 patients will be randomized to receive stem cell treatment or best medical management. Patients randomized to the medical management arm will eventually cross over to the stem cell arm.
Study participants must be age 18 or older and have experienced traumatic spinal cord injury within the past year. The spinal cord injuries must be American Spinal Injury Association (ASIA) grade A or B.
The initial participant in CELLTOP phase 1 sustained a C3-4 ASIA grade A spinal cord injury. As described in the February 2020 issue of Mayo Clinic Proceedings, the neurological examination at the time of the injury revealed complete loss of motor and sensory function below the level of injury.
After undergoing urgent posterior cervical decompression and fusion, as well as physical and occupational therapy, the patient demonstrated improvement in motor and sensory function. But that progress plateaued six months after the injury.
Stem cells were injected nearly a year after his injury and several months after his improvement had plateaued. Clinical signs of efficacy in both motor and sensory function were observed at three, six, 12 and 18 months following the stem cell injection.
"Our patient also reported a strong improvement with his grip and pinch strength, as well as range of motion for shoulder flexion and abduction," Dr. Bydon says.
Spinal cord injury has a complex pathophysiology. After the primary injury, microenvironmental changes inhibit axonal regeneration. Stem cells can potentially provide trophic support to the injured spinal cord microenvironment by modulating the inflammatory response, increasing vascularization and suppressing cystic change.
"In the phase 2 study, we will begin to learn the characteristics of individuals who respond to the therapy in terms of their age, severity of injury and time since injury," says Anthony J. Windebank, M.D., a neurologist at Mayo's campus in Minnesota and director of the Regenerative Neurobiology Laboratory. "We will also use biomarker studies to learn about the characteristics of responders' cells. The next phase would be studying how we can modify everyone's cells to make them more like the cells of responders."
CELLTOP illustrates Mayo Clinic's commitment to regenerative medicine therapies for neurological care. "Our findings to date will be encouraging to patients with spinal cord injuries," Dr. Bydon says. "We are hopeful about the potential of stem cell therapy to become part of treatment algorithms that improve physical function for patients with these devastating injuries."
Bydon M, et al. CELLTOP clinical trial: First report from a phase I trial of autologous adipose tissue-derived mesenchymal stem cells in the treatment of paralysis due to traumatic spinal cord injury. Mayo Clinic Proceedings. 2020;95:406.
Regenerative Neurobiology Laboratory: Anthony J. Windebank. Mayo Clinic.
Read the rest here:
Stem cell treatment after spinal cord injury: The next ...


 October 27th, 2020
October 27th, 2020